My Account
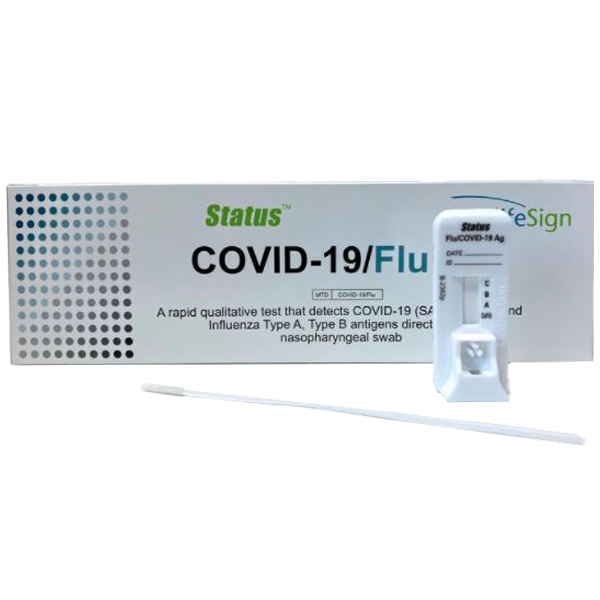
lifeSign Status™ COVID-19/Flu Test Kits in Bulk
COVID-19/Flu tests are the only FDA EUA visually read rapid test for simultaneous detection and differential diagnosis - no additional equipment needed! These tests are authorized for on-site settings operating under a CLIA certificate, and all tests that we carry have FDA Emergency Use Authorization (EUA). All tests should be administered by trained medical professionals. Ask us about large volume discounts and payment term options.
Get a Quick Quote
Enter your contact info and how many test kits you need:
Why lifeSign Status™?
- 25 Tests/Kit
- Results in 15 minutes
- 3 tests in 1 consolidates and speeds workflow
- No equipment (analyzer) needed
- COVID-19 Test - sensitivity of 93.1% and specificity of 100%
- Flu A - sensitivity of 91.4% and specificity of 95.7%
- Flu B - sensitivity of 87.6% and specificity of 95.9%
- Antigen test for use in anterior nasal and nasopharyngeal swab specimens
- For prescription use only
- For in vitro diagnostic use only
- Authorized for on-site settings operating under a CLIA certificate
- Store between 35.6-86°F / 2-30°C
- FDA Emergency Use Authorization (EUA) Approval Letter
- Extended Shelf Life
- Variant Revision Letter
- Instructions For Use
Frequently Asked Questions
What happens if I receive a positive result?
If you receive a positive result you should remain in self-isolation, and inform your healthcare provider of your result. You should follow the guidance provided by the Centers for Disease Control and Prevention (CDC).
If you develop emergency warning signs for COVID-19, get medical attention immediately. Emergency warning signs include*:
- Difficulty breathing or shortness of breath
- Persistent pain or pressure in the chest
- New confusion or inability to arouse
- Bluish lips or face *This list is not all inclusive. Please consult your medical provider for any other symptoms that are severe or concerning.
What does FDA authorized mean?
When there is a critical situation impacting health and safety and there are no FDA-approved or cleared tests available, and other criteria are met, the FDA can make tests available under an emergency access mechanism called an Emergency Use Authorization (EUA). The EUA for this test is supported by the Secretary of Health and Human Service’s (HHS’s) declaration that circumstances exist to justify the emergency use of in vitro diagnostics for the detection and / or diagnosis of the virus that causes COVID-19.
Select tests have been issued an Emergency Use Authorization (EUA) from the U.S. Food and Drug Administration (FDA) for prescription home use, which provides consumers with the ability to test themselves from the comfort and safety of their homes.
Select tests have been issued an Emergency Use Authorization (EUA) from the U.S. Food and Drug Administration (FDA) for prescription home use, which provides consumers with the ability to test themselves from the comfort and safety of their homes.
What does Sensitivity and Specificity mean?
In a diagnostic test, Sensitivity is a measure of how well a test can identify true positives. In a diagnostic test, Specificity is a measure of how well a test can identify true negatives.
How do I place a bulk order for my organization or institution?
For bulk order inquiries, please fill out the quote form at the top of this page with as much detail as possible pertaining to your request.
Why Buy COVID Test Kits for Your Business?
iPromo is proud to offer a huge selection of COVID testing options for businesses, government organizations and entities looking to slow the spread of COVID-19 within their organization. In order to return to business as usual, we must prevent super-spreader events and outbreaks wherever possible. Our bulk COVID test kit packages put testing control in the hands of your business, ensuring that your workplace or event isn’t a source of spread.
Point-Of-Care COVID Testing for Businesses
Keep your workplace or event safe and help control the spread of COVID-19 with iPromo’s bulk point-of-care COVID testing options. These convenient COVID antigen tests are specifically designed for on-site POC administration at conferences, events, meetings and more. Business owners and organizations should consider stocking up on bulk COVID tests for on-site use to prevent super spreader events and slow the spread within the community.
Each COVID test kit is designed to provide accurate detection of COVID in both symptomatic and asymptomatic people in as little as 10 minutes, depending on the kit you choose. Unlike our at-home COVID test kits, point-of-care tests must be administered by a medical professional or trained administrator. However, they don’t require you to send the tests to a lab for results. This makes them a convenient option for all sorts of events and applications, from testing guests at weddings and concerts to checking into medical facilities and schools.
Each COVID test kit is designed to provide accurate detection of COVID in both symptomatic and asymptomatic people in as little as 10 minutes, depending on the kit you choose. Unlike our at-home COVID test kits, point-of-care tests must be administered by a medical professional or trained administrator. However, they don’t require you to send the tests to a lab for results. This makes them a convenient option for all sorts of events and applications, from testing guests at weddings and concerts to checking into medical facilities and schools.
COVID Tests for Events and More
Each of these bulk COVID testing options has been approved by the U.S. Food and Drug Administration (FDA) with Emergency Use Authorization (EUA), so you can trust them to be safe and effective for your employees. Stock up on at-home COVID tests in bulk for your next sporting event, concert or conference or to ready your team to return to the office. iPromo is happy to supply you with a custom quote to suit your budget and needs.
More Test Kits:
Copyright © 1999-2024 All Rights Reserved. iPromo©