Bulk COVID Test Kits
At-home COVID-19 test solutions are convenient and easy to use in the privacy of your home, no need to go anywhere to get tested. We only carry tests that have FDA Emergency Use Authorization (EUA), so you can rest assured that you are getting the best solutions available. Ask us about large volume discounts and payment term options. Due to high demand, stock and delivery time may vary and are subject to change.
Now available - Flu, Strep and RSV test kits! Click here for all test kits
Get a Quick Quote
Enter your contact information and how many tests you need


We carry many different at-home test kits
so there is one to fit your specific needs
iHealth® COVID-19 Antigen Rapid Test – 2 pack
The iHealth® COVID-19 Antigen Rapid Test is an easy-to-use lateral flow assay authorized for non-prescription home use with a non-invasive nasal swab. The test can be completed in the comfort of your own home without the need to ship your sample to a lab. The self-administered test is recommended for individuals aged 15 years and older. Adult-collection is required for testing children 2-14 years old.


- Results in 15 minutes
- Test sensitivity of 94.3% and specifity of 98.1%
- FDA Emergency Use Authorization (EUA)
- Convenient at-home test, no prescription needed
- For in vitro diagnostic use only
- iHealth Test app with step-by-step instructional videos (app not required)
- Store between 36-86°F / 2-30°C
- Instructions for Use
- Read More About Extended Shelf-Life
- FDA Emergency Use Authorization (EUA) Approval Letter
- iPromo will only sell true FDA EUA approved tests (Click here to learn more.) FDA helps detect counterfeit at-home tests vs. real tests (Click here to learn more.)
Abbott BinaxNOW™ Home Card Test – 2 pack
If a Digital Health Pass is not necessary, or there is a tight budget, this Abbott BinaxNOW™ COVID-19 Rapid Antigen Self Test is ideal. It is an at-home solution authorized for non-prescription home use with self-collected direct anterior nasal (nares) swab samples from individuals 15 years or older, or 2 years or older only if samples are collected by an adult.

- Results reporting in 15 minutes
- Test sensitivity of 84.6% and specificity of 98.5%
- Detects the virus that contain variants, such as Omicron and Delta
- 2 tests in each box
- No need to send results to a lab
- Prescription is not needed
- Store between 35.6-86°F / 2-30°C
- Read More About Extended Shelf-Life
- Instructions for Use
- FDA Emergency Use Authorization (EUA) Approval Letter
Abbott BinaxNOW™ COVID-19 Ag Home Card Test Enabled by eMed – 1 pack
Abbott BinaxNOW™ COVID-19 Ag Card Home Test, Enabled by eMed, utilizes a digital platform to virtually guide the user through the test process from beginning to end. The test delivers results in just 15 minutes with no instrumentation, using proven lateral flow technology. eMed’s test includes a verified lab report in 15 minutes, online 24/7, no appointment needed. This CLIA-waived lab report means allows users to display a digital pass accepted by airlines and cruise lines for entry and reentry into the United States.


- Results reporting in 15 minutes
- Easy to use with a wifi and camera-enabled laptop or mobile phone
- Test sensitivity of 97.1% and specificity of 98.5%
- Detects the virus that contain variants, such as Omicron and Delta
- Third-party verification ensures authentication
- Online 24/7 lab report and digital pass for travel
- 24/7 Live Support – eMed certified guides & customer support agents
- Store between 35.6-86°F / 2-30°C
- Instructions for Use
- FDA Emergency Use Authorization (EUA) Approval Letter
Quidel QuickVue® At-Home OTC COVID-19 Test – 2 pack
Quidel is a trusted innovator in the medical device industry for over 40 years and is a leading provider of rapid diagnostic testing solutions. The QuickVue test has FDA Emergency Use Authorization (EUA) and employs the same Quidel lateral flow technology used for decades by healthcare professionals. It is an at-home solution intended to detect antigens from SARS-CoV-2 from individuals with or without symptoms when tested twice over two or three days, and with at least 24 hours and no more than 36 hours between tests. This test is authorized for individuals aged 14 years and older, and individuals ranging in ages 2 to 14 must have the test administered by an adult.


- Results in 10 minutes
- Excellent performance, with positive results agreeing with PCR test 83.5%, and negative results agreeing with PCR test 99.2% of the time
- Detects the virus that contain variants, such as Omicron and Delta
- Utilizes trusted technology by used for decades by healthcare professionals
- FDA Emergency Use Authorization (EUA)
- Store between 59-86°F / 15-30°C
- Instructions for Use
- FDA Emergency Use Authorization (EUA) Approval Letter
ACON® Flowflex™ COVID-19 Antigen Home Test – 1 pack
The Flowflex™ COVID-19 Antigen Home Test is is all you need to determine your family’s Covid-19 status, whether symptoms are present or not. Can be used on children as young as 2 years old. Get the convenience of Flowflex! iPromo will only sell the true FDA EUA Approved test in the white box (see image above), and not the test in the blue box which was recalled in the U.S. (Click here to learn more.)

- Reliable results in 15 minutes
- Test sensitivity of 93% and specificity of 100%
- Painless nasal sample swab
- No prescription required
- Authorized under FDA EUA, for home use
- Compact packaging for "On-the-Go" testing
- Store between 36-86°F (2-30°C)
- Instructions for Use
- Read More About Extended Shelf-Life
- FDA Emergency Use Authorization (EUA) Approval Letter
- iPromo will only sell the true FDA EUA Approved test in the white box (see image above), and not the test in the blue box which was recalled in the U.S. (Click here to learn more.) FDA helps detect counterfeit at-home tests vs. real tests (Click here to learn more.)



AccessBio CareStart™ COVID-19 Antigen Home Test – 2 pack
The CareStart™ COVID-19 Antigen Home Test is authorized for non-prescription home use with self-collected anterior nasal (nares) swab samples from individuals aged 14 years or older with symptoms of COVID-19 within the first 7 days of symptom onset. This test is also authorized for non-prescription home use with adult-collected nasal (nares) swab samples from individuals aged 2 years or older with symptoms of COVID-19 within the first 7 days of symptom onset.
- Reliable results in 10 minutes
- Great performance--87% sensitivity and 98% specificity
- Detects the virus that contain variants, such as Omicron and Delta
- Emergency Use Authorization (EUA) received
- Prescription is not needed
- For in vitro diagnostic use only
- Install required on/go™ mobile application that offers self-paced step-by-step directions
- Made in the USA
- Store between 34-86°F (1-30°C)
- Instructions for Use
- Read More About Extended Shelf-Life
- FDA Emergency Use Authorization (EUA) Approval Letter


Intrivo™ on/go™ COVID-19 Antigen Rapid Self-Test – 2 pack
The on/go™ COVID test is a portable, reliable and affordable over-the-counter, self-administered rapid antigen test that delivers results in just ten minutes, with 95% accuracy. on/go™ can be used to detect the antigen proteins from all major known COVID-19 variants of concern, like Omicron.


- Results reporting in 10 minutes
- Great performance--87% sensitivity and 98% specificity
- Detects the virus that contain variants, such as Omicron and Delta
- Emergency Use Authorization (EUA) received
- Install required on/go™ mobile application that offers
self-paced step-by-step directions
- Prescription is not needed
- For in vitro diagnostic use only
- Store between 34-86°F / 1-30°C
- Made in the USA
- Instructions for Use
- FDA Emergency Use Authorization (EUA) Approval Letter


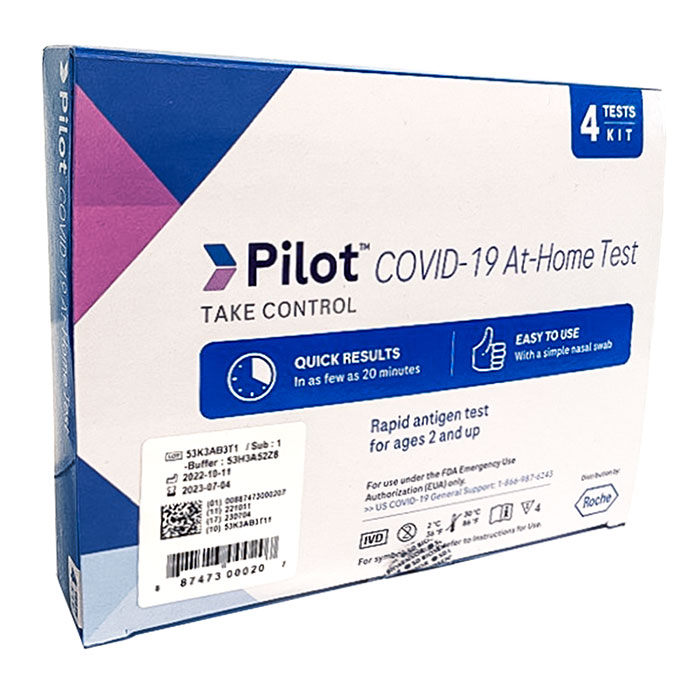
Roche Pilot™ COVID-19 At-Home Test – 4 pack
The accuracy of the Pilot COVID-19 At-Home Test provides you with a result you can trust and the confidence you need to take control. The Pilot COVID-19 At-Home Test is an easy-to-use, accurate and reliable rapid antigen test that delivers results in as few as 20 minutes. Approved for the detection of COVID-19 with or without symptoms for ages 2 and up. Adult collection required for children 2-13 years old. The package of four tests easily allows for family and/or serial testing. FSA/HSA eligible. The Pilot COVID-19 At-Home Test detects all known variants of COVID-19, including the Omicron and Delta variants.


- Results in 20 minutes
- Great performance--93.2% sensitivity and 100% specificity
- Detects the virus that contain variants, such as Omicron and Delta
- Emergency Use Authorization (EUA) received
- Prescription is not needed
- For in vitro diagnostic use only
- Store between 36-86°F / 2-30°C
- Instructions for Use
- FDA Emergency Use Authorization (EUA) Approval Letter
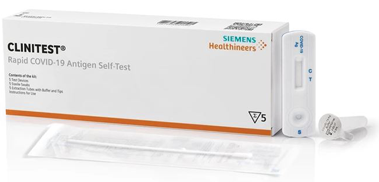
Healgen Siemens® Healthineers CLINITEST® COVID-19 Antigen Self-Test – 1 pack
The CLINITEST® Rapid COVID-19 Antigen Self-Test is a lateral flow test for the qualitative detection of nucleocapsid protein antigen from SARS-CoV-2 (Coronavirus, or “COVID-19”) from direct anterior nasal (nares) swab. Nasal swab samples from individuals aged below 12 years or above 70 years should be collected by or under supervision of adults. This test is intended to aid in the rapid diagnosis of Coronavirus infections. If symptoms persist despite negative test results, it is recommended to visit a healthcare professional.

- Results in 15 minutes
- Test sensitivity of 86.5% and specifity of 99.3%
- Detects the virus that contain variants, such as Omicron and Delta
- Emergency Use Authorization (EUA) received
- Prescription is not needed
- For in vitro diagnostic use only
- Store between 36-86°F / 2-30°C
- Instructions
- FDA Emergency Use Authorization (EUA) Approval Letter

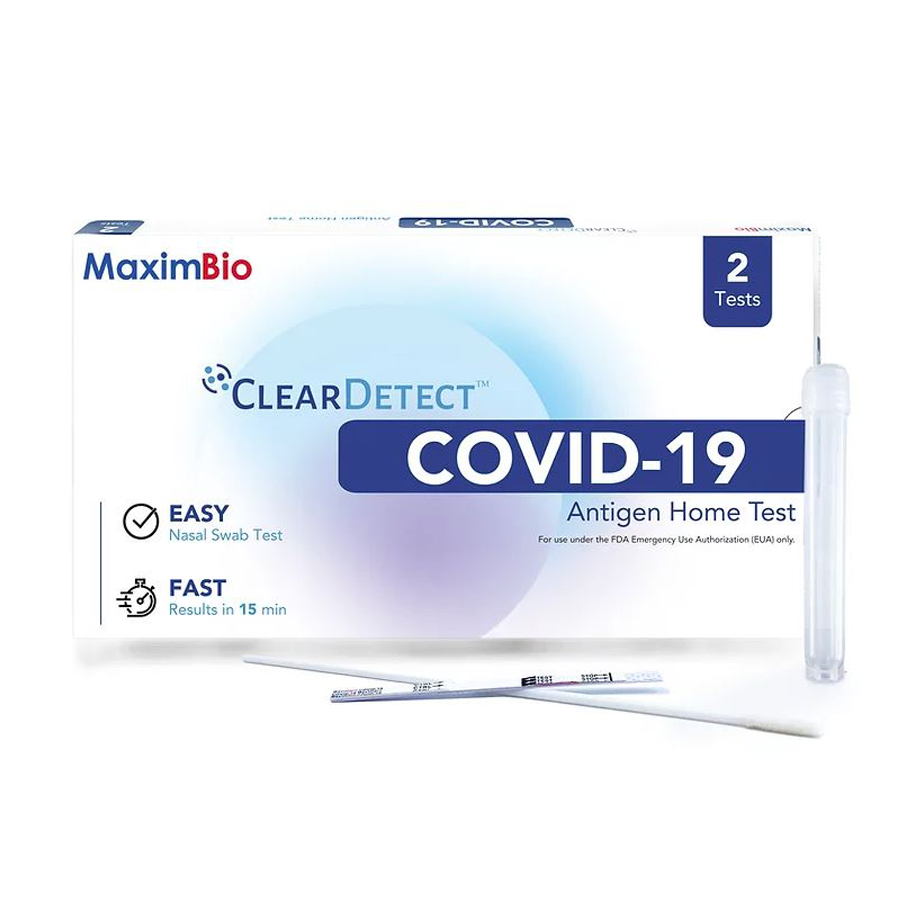
MaximBio ClearDetect™ COVID-19 Antigen Home Test – 2 pack
The MaximBio ClearDetect™ COVID-19 Antigen Home Test is authorized for non-prescription home use with self-collected anterior nasal swab samples from individuals aged 14 years or older, or adult-collected anterior nasal samples from individuals aged 2 or older, with or without symptoms or other epidemiological reasons to suspect COVID-19 when tested twice over three days with at least 24 hours (and no more than 48 hours) between tests.
- Results in 15 minutes
- Detects the virus that contain variants, such as Omicron and Delta
- For in vitro diagnostic use only
- Store between 39.2-86°F / 4-30°C
- Test sensitivity of 86.9% and specificity of 98.9%
- Convenient at-home test, no prescription needed
- Instructions for Use
- FDA Emergency Use Authorization (EUA) Approval Letter

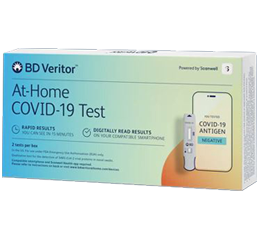
BD Veritor™ At-Home COVID-19 Test – 2 pack
The BD Veritor™ At-Home COVID-19 Test can be obtained without a prescription by individuals with or without symptoms or other epidemiological reasons to suspect COVID-19 when tested twice over two or three days with at least 24 hours and no more than 48 hours between tests.

- Results in 15 minutes
- Test sensitivity of 86.9% and specificity of 98.9%
- Detects the virus that contain variants, such as Omicron and Delta
- Digitally guided Scanwell® Health app
- Convenient at-home test, no prescription needed
- For in vitro diagnostic use only
- Store between 35-86°F / 2-30°C
- Instructions for Use
- FDA Emergency Use Authorization (EUA) Approval Letter


Which Test is Right For Your Organization
| Test Kit | Use Case | Tests/Kit | Reagents/Kit | FDA | Digital Health Pass | Prescription Required | Administer | Results |
|---|---|---|---|---|---|---|---|---|
| iHealth® COVID-19 Antigen Rapid Test | Home Use | 2 | 2 |  |
Self | 15 minutes | ||
| Abbott BinaxNOW™ Home Card Test | Home & Onsite: Workplaces, Consumers | 2 | 2 |  |
Self | 15 minutes | ||
| Abbott BinaxNOW™ COVID-19 Ag Home Card Test Enabled by eMed – 1 pack | Home & Onsite: Where Digital Health Pass is required: Travel, Workplaces, Events, Education | 1 | 1 |  |
 |
 |
Virtual Guided - Self | 15 minutes |
| Quidel QuickVue® At-Home OTC COVID-19 Test – 2 pack | Home Use | 2 | 2 |  |
Self | 10 minutes | ||
| ACON® Flowflex™ COVID-19 Antigen Home Test – 1 pack | Home Use | 1 | 1 |  |
Self | 15 minutes | ||
| AccessBio CareStart™ COVID-19 Antigen Home Test – 2 pack | Home Use | 2 | 2 |  |
Self | 10 minutes | ||
| Intrivo™ on/go™ COVID-19 Antigen Rapid Self-Test – 2 pack | Home Use | 2 | 2 |  |
Self | 10 minutes | ||
| Roche PilotTM COVID-19 At-Home Test – 4 pack | Home Use | 4 | 4 |  |
Self | 20 minutes | ||
| Healgen Siemens® Healthineers CLINITEST® COVID-19 Antigen Self-Test – 1 pack | Home Use | 5 | 5 |  |
Self | 15 minutes | ||
| MaximBio ClearDetect™ COVID-19 Antigen Home Test | Home Use | 2 | 2 |  |
Self | 15 minutes | ||
| BD Veritor™ At-Home COVID-19 Test | Home Use | 2 | 2 |  |
Self | 15 minutes |
Frequently Asked Questions
If you develop emergency warning signs for COVID-19, get medical attention immediately. Emergency warning signs include*:
- Difficulty breathing or shortness of breath
- Persistent pain or pressure in the chest
- New confusion or inability to arouse
- Bluish lips or face *This list is not all inclusive. Please consult your medical provider for any other symptoms that are severe or concerning.
Select tests have been issued an Emergency Use Authorization (EUA) from the U.S. Food and Drug Administration (FDA) for prescription home use, which provides consumers with the ability to test themselves from the comfort and safety of their homes.
- iHealth® COVID-19 Antigen Rapid Test: Do not use on anyone under the age of 2 years of age. Test takers ages 2 through 14 must be tested by an adult
- Abbott BinaxNOW™ Home Test: For use of individuals 15 years or older, or ages 2-14 only if samples are collected by an adult
- Abbott BinaxNOW™ Home Test with Digital Health Pass: For use of individuals 15 years or older, ages 2-14 must have an adult present during specimen collection. Must be at least 18yrs to order.
- Quidel QuickVue® At-Home OTC COVID-19 Test: Test authorized for use by persons age 2+; Test takers ages 2 through 14 must be accompanied by an adult during specimen collection
- ACON® Flowflex™ COVID-19 Antigen Home Test: Do not use on anyone under two years of age. A nasal swab sample can be self-collected by an individual age or older. Children aged 2 to 13 should be tested by an adult.
- AccessBio CareStart™ COVID-19 Antigen Home Test: Do not use on anyone under 2 years of age. This test is authorized for non-prescription home use with self-collected direct anterior nasal (nares) swab samples from individuals aged 14 years or older or adult collected anterior nasal swab samples from individuals aged 2 years or older.
- Intrivo™ on/go™ COVID-19 Antigen Rapid Self Test: Do not use on anyone under 2 years of age. This test is authorized for non-prescription home use with self-collected direct anterior nasal (nares) swab samples from individuals aged 14 years or older or adult collected anterior nasal swab samples from individuals aged 2 years or older.
- Roche Pilot™ COVID-19 At-Home Test: Do not use on anyone under two years of age. A nasal swab sample can be self-collected by an individual age or older. Children aged 2 to 13 should be tested by an adult.
- Healgen Siemens® Healthineers CLINITEST® COVID-19 Antigen Self-Test: Do not use on anyone under two years of age. An anterior nasal swab sample can be self-collected by an individual age or older. Children aged 2 to 13 should be tested by an adult.
- MaximBio ClearDetect™ COVID-19 Antigen Home Test: Do not use on anyone under two years of age. An anterior nasal swab sample can be self-collected by an individual age or older. Children aged 2 to 13 should be tested by an adult.
The beauty of these easy-to-use home self-tests is that they do not require a medical professional to administer. With rapid reporting in 15 minutes and no need to send results to a lab, users can easily test themselves without hassle. For example, the Abbott BinaxNOW™ COVID-19 Antigen Self-Test and others come with easy-to-follow instructions and comfortable swabs for effective use at home or in the workplace.